NewsRescue
While today as it appears vaccines are being mandated in New York and other States, in May of this year New York Governor Andrew Cuomo categorically declared that by law vaccines cannot be directly or indirectly mandated. Speaking at a Covid briefing held May 4th 2021, Governor Cuomo declared:
“My agenda is very simple. I want to incentivize as many New Yorkers as possible to get vaccinated.”
Emphasizing that incentivizing vaccines was the best route to immunizing the unvaccinated New Yorkers because he cannot legally mandate vaccines.
Cuomo continued:
“You cannot mandate vaccines because the vaccines are approved under something called an emergency use authorization, EUA, and by law, you can’t mandate a vaccine approved under an EUA,” Gov. Cuomo said. “So you can’t, say for example, college students must have a vaccine. You cannot mandate a vaccine under an EUA. You can mandate measles which had a full approval, but you can’t mandate these vaccines which are still all under emergency use authorization.”
Today in violation of the law, New York state has mandated vaccines for New York employees, students and even restaurant and public event attendees.
See: Students ask Supreme Court to block college vaccine mandate
The legal repercussions of the flaunt of established laws under the United States constitution that prohibit mandating emergency use vaccines and the torrent of cases of class action suits against agencies and the government is being observed.
The Law is Clear on EUAs
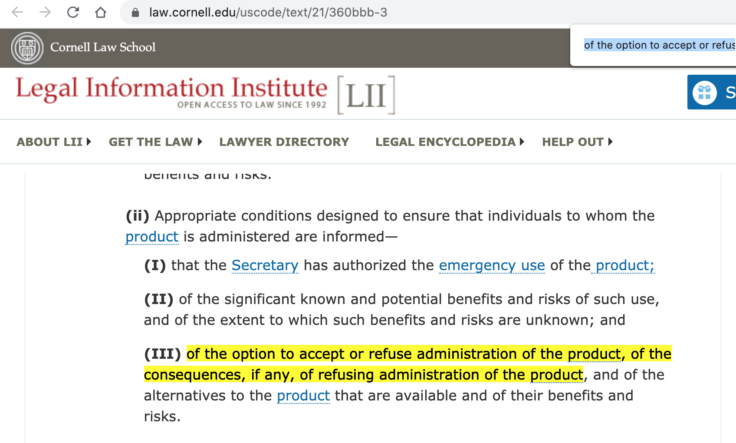
‘The same section of the Federal Food, Drug, and Cosmetic Act that authorizes the FDA to grant emergency use authorization also requires the secretary of Health and Human Services to “ensure that individuals to whom the product is administered are informed … of the option to accept or refuse administration of the product.”’
‘Likewise, the FDA’s guidance on emergency use authorization of medical products requires the FDA to “ensure that recipients are informed to the extent practicable given the applicable circumstances … That they have the option to accept or refuse the EUA product …”’ Read more
All Current Vaccines Are Yet To Get Full Approval
“Pfizer and BioNTech submitted their request for full approval, called a biologics license application, on May 7, and Moderna began a rolling submission in June. Johnson & Johnson has said it will submit its application later this year. Typically, it takes the agency at least several months to grant a full approval for a vaccine. But some officials have said that the Pfizer vaccine could be approved by late summer or early fall.” Read more
It has also been established that the enforced administration of Covid experimental vaccines evidently violate practically all 1947 Nuremberg Code international rules which guide the use of experimental interventions based on “The judgment by the war crimes tribunal at Nuremberg laid down 10 standards to which physicians must conform when carrying out experiments on human subjects in a new code that is now accepted worldwide.“
The Nuremberg Code (1947)
Permissible Medical Experiments
The great weight of the evidence before us to effect that certain types of medical experiments on human beings, when kept within reasonably well-defined bounds, conform to the ethics of the medical profession generally. The protagonists of the practice of human experimentation justify their views on the basis that such experiments yield results for the good of society that are unprocurable by other methods or means of study. All agree, however, that certain basic principles must be observed in order to satisfy moral, ethical and legal concepts:
- 1. The voluntary consent of the human subject is absolutely essential. This means that the person involved should have legal capacity to give consent; should be so situated as to be able to exercise free power of choice, without the intervention of any element of force, fraud, deceit, duress, overreaching, or other ulterior form of constraint or coercion; and should have sufficient knowledge and comprehension of the elements of the subject matter involved as to enable him to make an understanding and enlightened decision. This latter element requires that before the acceptance of an affirmative decision by the experimental subject there should be made known to him the nature, duration, and purpose of the experiment; the method and means by which it is to be conducted; all inconveniences and hazards reasonably to be expected; and the effects upon his health or person which may possibly come from his participation in the experiment.
The duty and responsibility for ascertaining the quality of the consent rests upon each individual who initiates, directs, or engages in the experiment. It is a personal duty and responsibility which may not be delegated to another with impunity.
2. The experiment should be such as to yield fruitful results for the good of society, unprocurable by other methods or means of study, and not random and unnecessary in nature.
3. The experiment should be so designed and based on the results of animal experimentation and a knowledge of the natural history of the disease or other problem under study that the anticipated results justify the performance of the experiment.
4. The experiment should be so conducted as to avoid all unnecessary physical and mental suffering and injury.
5. No experiment should be conducted where there is an a priori reason to believe that death or disabling injury will occur; except, perhaps, in those experiments where the experimental physicians also serve as subjects.
6. The degree of risk to be taken should never exceed that determined by the humanitarian importance of the problem to be solved by the experiment.
7. Proper preparations should be made and adequate facilities provided to protect the experimental subject against even remote possibilities of injury, disability or death.
8. The experiment should be conducted only by scientifically qualified persons. The highest degree of skill and care should be required through all stages of the experiment of those who conduct or engage in the experiment.
9. During the course of the experiment the human subject should be at liberty to bring the experiment to an end if he has reached the physical or mental state where continuation of the experiment seems to him to be impossible.
10. During the course of the experiment the scientist in charge must be prepared to terminate the experiment at any stage, if he has probable cause to believe, in the exercise of the good faith, superior skill and careful judgment required of him, that a continuation of the experiment is likely to result in injury, disability, or death to the experimental subject.





